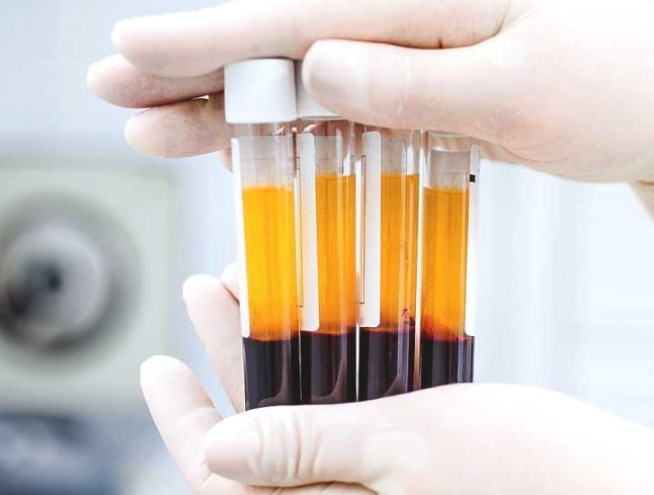
Possible Treatments: Convalescent Plasma (CP) Therapy
Convalescent plasma (CP) therapy has been used in the past to combat SARS, MERS, H1N1, Ebola and other viral infections. As a result, it is no wonder why CP therapy clinical trials are currently underway in many parts of the world to combat COVID-19. In this post, we will explain what CP therapy is, how it works, and its current applications in the COVID-19 pandemic.
What is CP therapy?
CP therapy is a blood-derived treatment that transfuses antibody-rich blood plasma from a recovered donor to a current patient. Scientists in China have been able to test this treatment in cell cultures (for an explanation on cell cultures, check out our last blog post) with results showing that the antibodies to be used in CP therapy could successfully neutralize the SARS-CoV-2 virus.
What is blood plasma and what makes it an effective therapy?
Blood plasma makes up about 55% of the total blood volume in a person’s body. It is the liquid component of the blood that is composed primarily of water (~95%) and contains many vital dissolved molecules such as glucose, oxygen, proteins, electrolytes, immune factors and more.
Blood plasma carries these essential molecules throughout the entire body.
Most relevant to the convalescent plasma therapy is the fact that plasma contains antibodies. Antibodies are proteins that are produced by our body's B cells, specifically plasma cells, when the immune system is exposed to a pathogen (in this case, the SARS-CoV-2 virus). Antibodies developed by the immune system are unique to each pathogen.
Once produced, the antibodies will attach themselves to the invading pathogen and then signal to the body’s immune system to attack and remove the intruder as quickly as possible.
These antibodies do not remain in our blood indefinitely (having a half-life of 5-21 days), but luckily our body has memory B cells which will remember the specific pathogen’s invasion and keep the blueprints for that corresponding antibody so that the immune system can quickly produce them if exposed to the same pathogen.
Scientists have noted two (of five) types of antibodies that have been tested in the COVID-19 CP therapies: IgM and IgG.
IgM antibodies are the ‘first line’ of antibodies and are most active in early infection until a sufficient amount of IgG antibodies are produced. IgG antibodies make up a majority of the antibodies in our blood and do the most work to neutralize (attack and remove) the SARS-CoV-2 virus during the infection.
How does the CP therapy work?
Blood is collected from recovered COVID-19 patients who have been symptom-free for at least 14 days prior to donation. Donors and recipients must have compatible blood types in order to successfully receive the convalescent plasma transfusion.
Blood plasma is separated from the other components of the blood through a process called centrifugation.
Centrifugation is a laboratory technique used to separate particles in a mixture according to weight, density, or particle size. When blood is centrifuged, plasma, which is the least dense component of blood, will sit atop the other components and can then be isolated.
In order for the therapy to be effective, the donor must have a certain level of SARS-CoV-2 specific antibody titer in their blood. Antibody titers are usually used to check the strength of a person’s immune response, the progression of an autoimmune disorder, the need for a booster shot of a vaccine, or any recent infections.
Antibody titers are represented as ratios that tell us how many times the blood serum (extract) can be diluted and still have detectable levels of the antibody. The FDA recommends that for the CP therapy in COVID-19, the antibody titer should be at least 1:160. This ratio means that the blood serum from a recovered patient should be able to be diluted, in a ratio of 1 part blood serum to 160 parts diluent, and still test positive for the SARS-CoV-2 specific antibodies.
Once the plasma is separated with the appropriate antibody titer, it can then be transfused into patients fighting the SARS-CoV-2 virus. Scientists believe this transfusion can boost or supplement the immune system in patients who are having difficulty fighting off the virus. You can think about it like jump-starting a car when the battery is dead!
Success thus far…
*As a note, in most published cases, most patients were also receiving other antiviral drugs, however scientists believe that CP treatments contributed to the COVID-19 recovery.*
Most trials being conducted are only testing this therapy in critically ill patients or those with severe complications, however, many more clinical trials are still to come! In this trial in China, a majority of patients saw an improvement in their health with patients recovering and being discharged or taken off mechanical ventilators. Additionally, in this article from Korea, two elderly and critically-ill patients recovered and were discharged from the hospital after receiving CP therapy. For more, check the links below ‘In the News’.
In the News…
FDA announces clinical trial of CP therapy: Coronavirus (COVID-19) Update: FDA Coordinates National Effort to Develop Blood-Related Therapies for COVID-19
China: Blood from recovered coronavirus victims helps patient come off ventilator in just two days
United Kingdom: Wales' biggest hospital first in UK to carry out 'antibody transfusion' treatment on coronavirus patients
Journal Sources:
https://jamanetwork.com/journals/jama/fullarticle/2763983
https://jamanetwork.com/journals/jama/fullarticle/2763982
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4781783/
https://www.bmj.com/content/368/bmj.m1256
Media Sources:
https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
https://www.ucsfhealth.org/medical-tests/003333
https://ruo.mbl.co.jp/bio/e/support/method/antibody-isotype.html
Image Sources:
https://www.khanacademy.org/science/biology/human-biology/circulatory-pulmonary/a/components-of-the-blood
https://www.aboutkidshealth.ca/Article?contentid=927&language=English
https://www.straight.com/covid-19-pandemic/national-treatment-trial-using-blood-plasma-from-covid-19-survivors-to-start-in-hospitals
https://www.genscript.com/Western-Blot-Antibody-Troubleshooting.html